Articles
- Page Path
- HOME > Ann Coloproctol > Volume 40(3); 2024 > Article
-
Original Article
Immediate sphincter repair following fistulotomy for anal fistula: does it impact the healing rate and septic complications? -
Maher A. Abbas1
 , Anna T. Tsay2
, Anna T. Tsay2 , Mohammad Abbass3
, Mohammad Abbass3
-
Annals of Coloproctology 2024;40(3):217-224.
DOI: https://doi.org/10.3393/ac.2022.01144.0163
Published online: June 28, 2024
1Department of Surgery, King’s College Hospital Dubai, Dubai, United Arab Emirates
2Department of Surgery, Kaiser Permanente, Los Angeles, CA, USA
3Department of Surgery, Northwestern University, Chicago, IL, USA
- Correspondence to: Maher A. Abbas, MD, FACS, FASCRS Department of Surgery, King’s College Hospital Dubai, Dubai Hills, Dubai, United Arab Emirates Email: 'drmaherabbasmd@gmail.com'
© 2024 The Korean Society of Coloproctology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 612 Views
- 97 Download
Abstract
-
Purpose
- Fistulotomy is considered the most effective treatment for anal fistula; however, it carries a risk of incontinence. Sphincteroplasty in the setting of fistulotomy is not standard practice due to concerns regarding healing and potential infectious complications. We aimed to compare the outcomes of patients who underwent fistulotomy with primary sphincteroplasty to those who did not undergo repair.
-
Methods
- This was a retrospective review of consecutive patients who underwent fistulotomy for cryptoglandular anal fistula. All operations were performed by one colorectal surgeon. Sphincteroplasty was performed for patients perceived to be at higher risk for continence disturbance. The main outcome measures were the healing rate and postoperative septic complications.
-
Results
- In total, 152 patients were analyzed. Group A (fistulotomy with sphincteroplasty) consisted of 45 patients and group B (fistulotomy alone) included 107 patients. Both groups were similar in age (P=0.16) and sex (P=0.20). Group A had higher proportions of multiple fistulas (26.7% vs. 6.5%, P<0.01) and complex fistulas (mid to high transsphincteric, 37.8% vs. 10.3%; P<0.01) than group B. The median follow-up time was 8 weeks. The overall healing rate was similar in both groups (93.3% vs. 90.6%, P=0.76). No significant difference between the 2 groups was noted in septic complications (6.7% vs. 3.7%, P=0.42).
-
Conclusion
- Fistulotomy with primary sphincter repair demonstrated a comparable healing rate to fistulotomy alone, without an increased risk of postoperative septic complications. Further prospective randomized studies are needed to confirm these findings and to explore the functional outcomes of patients who undergo sphincteroplasty.
INTRODUCTION
METHODS
RESULTS
DISCUSSION
-
Conflict of interest
No potential conflict of interest relevant to this article was reported.
-
Funding
None.
-
Author contributions
Conceptualization: all authors; Formal analysis: ATT, MA; Investigation: ATT, MA; Methodology: all authors; Resources: ATT, MA; Writing–original draft: MAA; Writing–review & editing: all authors. All authors read and approved the final manuscript.
NOTES
| Study | Study type | No. of patients | Mean follow-up (mo) | Healing rate (%) | Sphincter repair dehiscence (%) | Septic complication (%) | Postoperative continence disturbance (%) |
|---|---|---|---|---|---|---|---|
| Parkash et al. [24] (1985)] | Retrospective | 120 | 6–60a | 96.6 | 11.7 | - | 0 |
| Christiansen and Rønholt [25] (1995) | Prospective | 14 | 12–48a | 85.7 | - | - | 21.4 |
| Gemsenjäger [26] (1996) | Retrospective | 21 | 2–9a | 95.2 | 4.8 | - | 4.8 |
| Toccaceli et al. [27] (1997) | Retrospective | 36 | 12 | - | 8.2 | - | 0 |
| Roig et al. [28] (1999) | Retrospective | 31 | 24b | 90.3 | 3.2 | 3.2 | 24.0 |
| Perez et al. [29] (2005) | Prospective | 35 | 32 | 94.3 | 0 | 0 | 12.5 |
| Perez et al. [30] (2006) | RCT | 28 | 36 | 92.9 | 0 | 0 | 17.4 |
| Jivapaisarnpong [31] (2009) | Prospective | 33 | 14 | 87.9 | - | 6.1 | 0 |
| Roig et al. [32] (2010) | Retrospective | 75 | 13 | 89.4 | 1.3 | - | 21.3 |
| Kraemer and Picke [33] (2011) | Retrospective | 38 | - | 97.4 | 2.6 | - | 5.3 |
| Arroyo et al. [34] (2012) | Retrospective | 70 | 81 | 91.5 | 0 | 1.4 | 16.6 |
| Ratto et al. [35] (2013) | Retrospective | 72 | 29.4 | 95.8 | 1.4 | 0 | 11.6 |
| Hirschburger et al. [36] (2014) | Retrospective | 50 | 22 | 88.0 | - | - | 6.0 |
| Seyfried et al. [37] (2018) | Retrospective | 424 | 11 | 88.2 | 7.5 | - | 23.0 |
| Litta et al. [38] (2019) | Retrospective | 203 | 56 | 93.0 | 1.4 | 0 | 13.0 |
| Farag et al. [39] (2019) | Retrospective | 175 | 12 | 90.9 | - | - | 2.3 |
| De Hous et al. [40] (2021) | Retrospective | 24 | 6b | 95.8 | 25.0 | - | 20.8 |
| Aguilar-Martínez et al. [41] (2021) | Retrospective | 107 | 96b | 84.1 | - | - | 14.9 |
| Orban et al. [42] (2021) | Retrospective | 24 | 6 | 83.3 | 8.3 | 16.7 | 12.5 |
| Jain et al. [43] (2022) | Prospective | 35 | 6 | 88.6 | - | 20 | 5.7 |
| This study (2023) | Retrospective | 45 | 2b | 93.3 | 0 | 6.7 | 17.8 |
- 1. Abbass MA, Abbas MA. Causes of operative failure. In: Abcarian H, editor. Anal fistula: principles and management. 1st ed. Springer; 2014. p. 177–89.Article
- 2. Abbas MA, Jackson CH, Haigh PI. Predictors of outcome for anal fistula surgery. Arch Surg 2011;146:1011–6.ArticlePubMed
- 3. Pastor C, Hwang J, Garcia-Aguilar J. Fistulotomy. Semin Colon Rectal Surg 2009;20:18–23.Article
- 4. Hyman N, O'Brien S, Osler T. Outcomes after fistulotomy: results of a prospective, multicenter regional study. Dis Colon Rectum 2009;52:2022–7.ArticlePubMed
- 5. van Koperen PJ, Wind J, Bemelman WA, Bakx R, Reitsma JB, Slors JF. Long-term functional outcome and risk factors for recurrence after surgical treatment for low and high perianal fistulas of cryptoglandular origin. Dis Colon Rectum 2008;51:1475–81.ArticlePubMed
- 6. Cavanaugh M, Hyman N, Osler T. Fecal incontinence severity index after fistulotomy: a predictor of quality of life. Dis Colon Rectum 2002;45:349–53.ArticlePubMed
- 7. Bokhari S, Lindsey I. Incontinence following sphincter division for treatment of anal fistula. Colorectal Dis 2010;12:e135–9.ArticlePubMed
- 8. Jordán J, Roig JV, García-Armengol J, García-Granero E, Solana A, Lledó S. Risk factors for recurrence and incontinence after anal fistula surgery. Colorectal Dis 2010;12:254–60.ArticlePubMed
- 9. Buchanan GN, Bartram CI, Phillips RK, Gould SW, Halligan S, Rockall TA, et al. Efficacy of fibrin sealant in the management of complex anal fistula: a prospective trial. Dis Colon Rectum 2003;46:1167–74.ArticlePubMed
- 10. Johnson EK, Gaw JU, Armstrong DN. Efficacy of anal fistula plug vs. fibrin glue in closure of anorectal fistulas. Dis Colon Rectum 2006;49:371–6.ArticlePubMed
- 11. Lawes DA, Efron JE, Abbas M, Heppell J, Young-Fadok TM. Early experience with the bioabsorbable anal fistula plug. World J Surg 2008;32:1157–9.ArticlePubMedPDF
- 12. Safar B, Jobanputra S, Sands D, Weiss EG, Nogueras JJ, Wexner SD. Anal fistula plug: initial experience and outcomes. Dis Colon Rectum 2009;52:248–52.ArticlePubMed
- 13. Abbas MA, Lemus-Rangel R, Hamadani A. Long-term outcome of endorectal advancement flap for complex anorectal fistulae. Am Surg 2008;74:921–4.ArticlePubMedPDF
- 14. Soltani A, Kaiser AM. Endorectal advancement flap for cryptoglandular or Crohn's fistula-in-ano. Dis Colon Rectum 2010;53:486–95.ArticlePubMed
- 15. Abbas MA, Sherman MJ. Endorectal advancement flap. In: Wexner SD, Fleshman JW, editors. Colon and rectal surgery: anorectal operations. 1st ed. Wolters Kluwer Health; 2012. p. 283–93.
- 16. Abcarian AM, Estrada JJ, Park J, Corning C, Chaudhry V, Cintron J, et al. Ligation of intersphincteric fistula tract: early results of a pilot study. Dis Colon Rectum 2012;55:778–82.ArticlePubMed
- 17. Tan KK, Alsuwaigh R, Tan AM, Tan IJ, Liu X, Koh DC, et al. To LIFT or to flap? Which surgery to perform following seton insertion for high anal fistula? Dis Colon Rectum 2012;55:1273–7.ArticlePubMed
- 18. Fitzpatrick M, Fynes M, Cassidy M, Behan M, O'Connell PR, O'Herlihy C. Prospective study of the influence of parity and operative technique on the outcome of primary anal sphincter repair following obstetrical injury. Eur J Obstet Gynecol Reprod Biol 2000;89:159–63.ArticlePubMed
- 19. Glasgow SC, Lowry AC. Long-term outcomes of anal sphincter repair for fecal incontinence: a systematic review. Dis Colon Rectum 2012;55:482–90.ArticlePubMed
- 20. Ratto C, Litta F, Donisi L, Parello A. Fistulotomy or fistulectomy and primary sphincteroplasty for anal fistula (FIPS): a systematic review. Tech Coloproctol 2015;19:391–400.ArticlePubMedPDF
- 21. Iqbal N, Dilke SM, Geldof J, Sahnan K, Adegbola S, Bassett P, et al. Is fistulotomy with immediate sphincter reconstruction (FISR) a sphincter preserving procedure for high anal fistula? A systematic review and meta-analysis. Colorectal Dis 2021;23:3073–89.ArticlePubMedPDF
- 22. Ratto C, Grossi U, Litta F, Di Tanna GL, Parello A, De Simone V, et al. Contemporary surgical practice in the management of anal fistula: results from an international survey. Tech Coloproctol 2019;23:729–41.ArticlePubMedPMCPDF
- 23. Gaertner WB, Burgess PL, Davids JS, Lightner AL, Shogan BD, Sun MY, et al. The American Society of Colon and Rectal Surgeons clinical practice guidelines for the management of anorectal abscess, fistula-in-ano, and rectovaginal fistula. Dis Colon Rectum 2022;65:964–85.ArticlePubMed
- 24. Parkash S, Lakshmiratan V, Gajendran V. Fistula-in-ano: treatment by fistulectomy, primary closure and reconstitution. Aust N Z J Surg 1985;55:23–7.ArticlePubMed
- 25. Christiansen J, Rønholt C. Treatment of recurrent high anal fistula by total excision and primary sphincter reconstruction. Int J Colorectal Dis 1995;10:207–9.ArticlePubMedPDF
- 26. Gemsenjäger E. Results with a new therapy concept in anal fistula: suture of the anal sphincter. Schweiz Med Wochenschr 1996;126:2021–5.PubMed
- 27. Toccaceli S, Minervini S, Salvio A, Zarba Meli E, Mazzocchi P, Lepiane P, et al. Fistulectomy with closure by first intention in the treatment of perianal fistulae. Minerva Chir 1997;52:377–81.PubMed
- 28. Roig JV, Garcia-Armengol J, Jordán J, Alos R, Solana A. Immediate reconstruction of the anal sphincter after fistulectomy in the management of complex anal fistulas. Colorectal Dis 1999;1:137–40.ArticlePubMed
- 29. Perez F, Arroyo A, Serrano P, Candela F, Sanchez A, Calpena R. Fistulotomy with primary sphincter reconstruction in the management of complex fistula-in-ano: prospective study of clinical and manometric results. J Am Coll Surg 2005;200:897–903.ArticlePubMedPDF
- 30. Perez F, Arroyo A, Serrano P, Sánchez A, Candela F, Perez MT, et al. Randomized clinical and manometric study of advancement flap versus fistulotomy with sphincter reconstruction in the management of complex fistula-in-ano. Am J Surg 2006;192:34–40.ArticlePubMed
- 31. Jivapaisarnpong P. Core out fistulectomy, anal sphincter reconstruction and primary repair of internal opening in the treatment of complex anal fistula. J Med Assoc Thai 2009;92:638–42.PubMed
- 32. Roig JV, García-Armengol J, Jordán JC, Moro D, García-Granero E, Alós R. Fistulectomy and sphincteric reconstruction for complex cryptoglandular fistulas. Colorectal Dis 2010;12:e145–52.ArticlePubMed
- 33. Kraemer M, Picke D. Fistulotomy with primary sphincter repair for the treatment of anal fistula. Coloproctology 2011;33:104–8.ArticlePDF
- 34. Arroyo A, Pérez-Legaz J, Moya P, Armañanzas L, Lacueva J, Pérez-Vicente F, et al. Fistulotomy and sphincter reconstruction in the treatment of complex fistula-in-ano: long-term clinical and manometric results. Ann Surg 2012;255:935–9.ArticlePubMed
- 35. Ratto C, Litta F, Parello A, Zaccone G, Donisi L, De Simone V. Fistulotomy with end-to-end primary sphincteroplasty for anal fistula: results from a prospective study. Dis Colon Rectum 2013;56:226–33.ArticlePubMed
- 36. Hirschburger M, Schwandner T, Hecker A, Kierer W, Weinel R, Padberg W. Fistulectomy with primary sphincter reconstruction in the treatment of high transsphincteric anal fistulas. Int J Colorectal Dis 2014;29:247–52.ArticlePubMedPDF
- 37. Seyfried S, Bussen D, Joos A, Galata C, Weiss C, Herold A. Fistulectomy with primary sphincter reconstruction. Int J Colorectal Dis 2018;33:911–8.ArticlePubMedPDF
- 38. Litta F, Parello A, De Simone V, Grossi U, Orefice R, Ratto C. Fistulotomy and primary sphincteroplasty for anal fistula: long-term data on continence and patient satisfaction. Tech Coloproctol 2019;23:993–1001.ArticlePubMedPDF
- 39. Farag AF, Elbarmelgi MY, Mostafa M, Mashhour AN. One stage fistulectomy for high anal fistula with reconstruction of anal sphincter without fecal diversion. Asian J Surg 2019;42:792–6.ArticlePubMed
- 40. De Hous N, Van den Broeck T, de Gheldere C. Fistulectomy and primary sphincteroplasty (FIPS) to prevent keyhole deformity in simple anal fistula: a single-center retrospective cohort study. Acta Chir Belg 2021;121:308–13.ArticlePubMed
- 41. Aguilar-Martínez MD, Sánchez-Guillén L, Barber-Valles X, Alcaide-Quirós MJ, Bosch-Ramírez M, López-Delgado A, et al. Long-term evaluation of fistulotomy and immediate sphincteroplasty as a treatment for complex anal fistula. Dis Colon Rectum 2021;64:1374–84.ArticlePubMed
- 42. Orban YA, Soliman HH, El Teliti AM, El-Shewy A, Hegab YH, Ibrahim A. Evaluation of fistulotomy with immediate sphincteric reconstruction in the treatment of high transsphincteric perianal fistula. J Coloproctol 2021;41:217–221.Article
- 43. Jain S, Baghel H, Ghanghoria A, Gupta S, Kelum L, Chaudhary P, et al. A prospective and comparative study on fistulotomy with sphincteroplasty or fistulotomy without sphincheteroplasty of partially damaged internal sphincter and assessment of incontinence, recurrence and patient satisfaction in the patient of high fistula-in-ano. Arch Clin Exp Surg 2022;11:1–6.PDF
- 44. Rodrigues FG, Chadi SA, Cracco AJ, Sands DR, Zutshi M, Gurland B, et al. Faecal incontinence in patients with a sphincter defect: comparison of sphincteroplasty and sacral nerve stimulation. Colorectal Dis 2017;19:456–61.ArticlePubMedPDF
- 45. Ommer A, Herold A, Berg E, Fürst A, Sailer M, Schiedeck T, et al. Cryptoglandular anal fistulas. Dtsch Arztebl Int 2011;108:707–13.ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations

 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
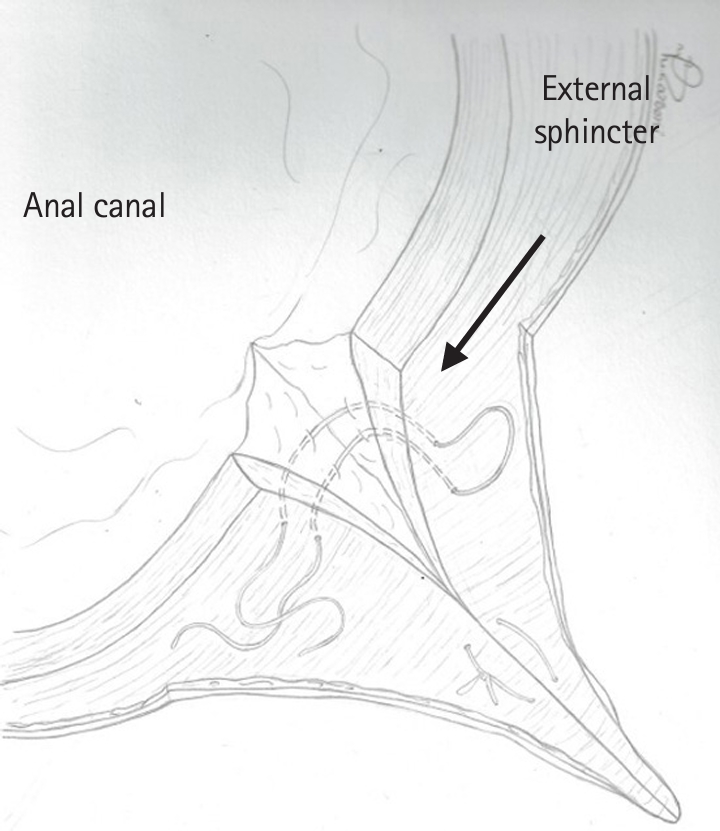
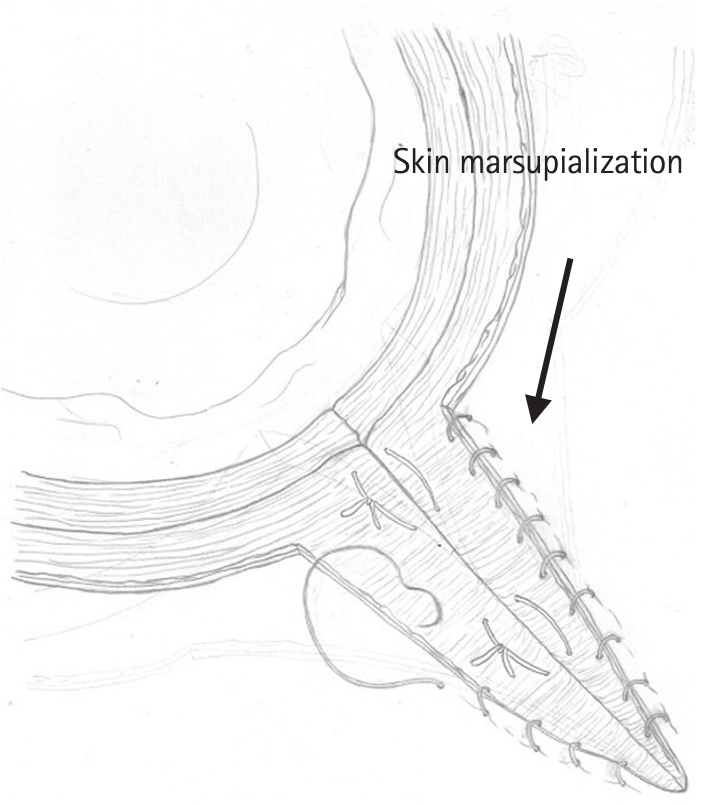
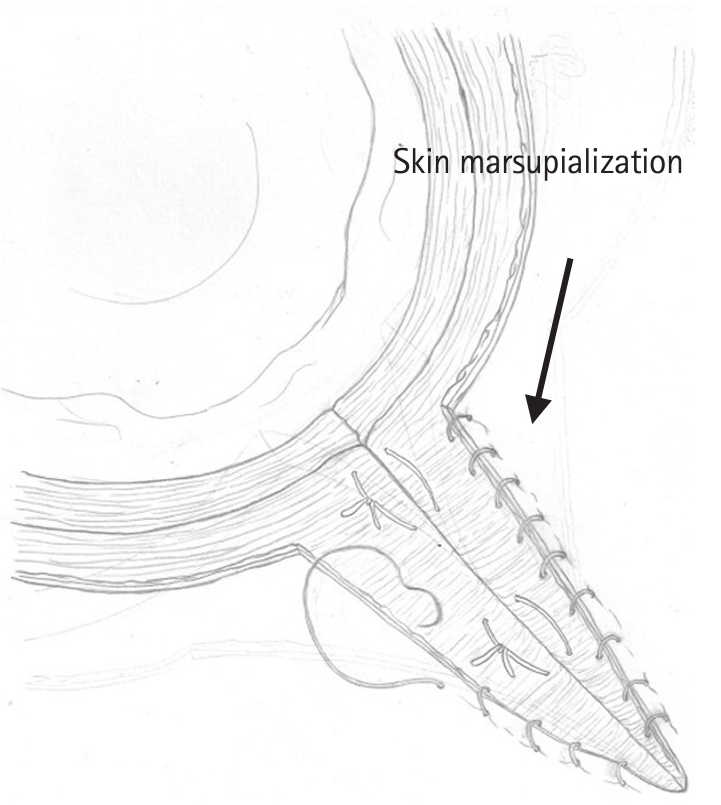
- Figure
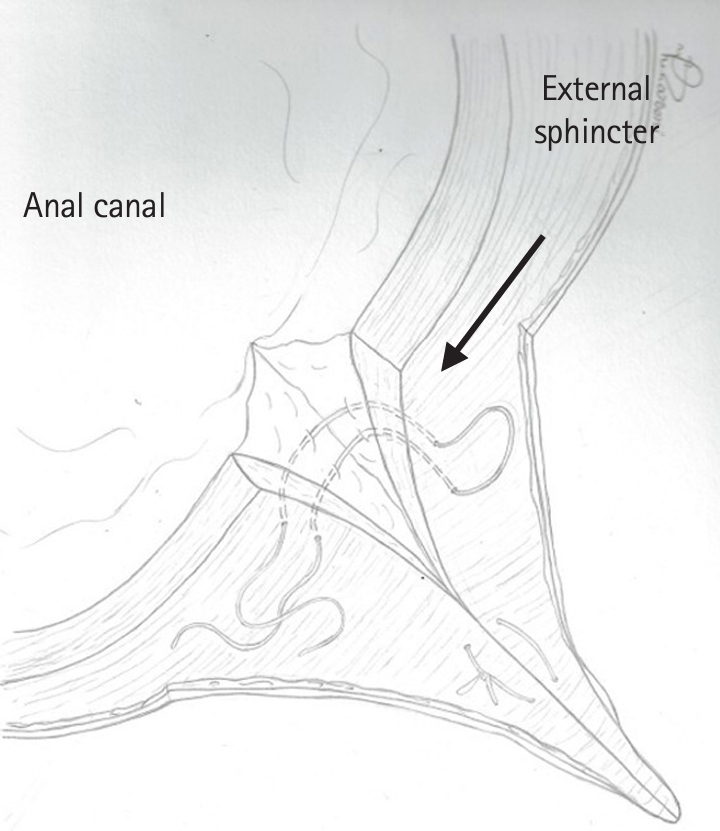
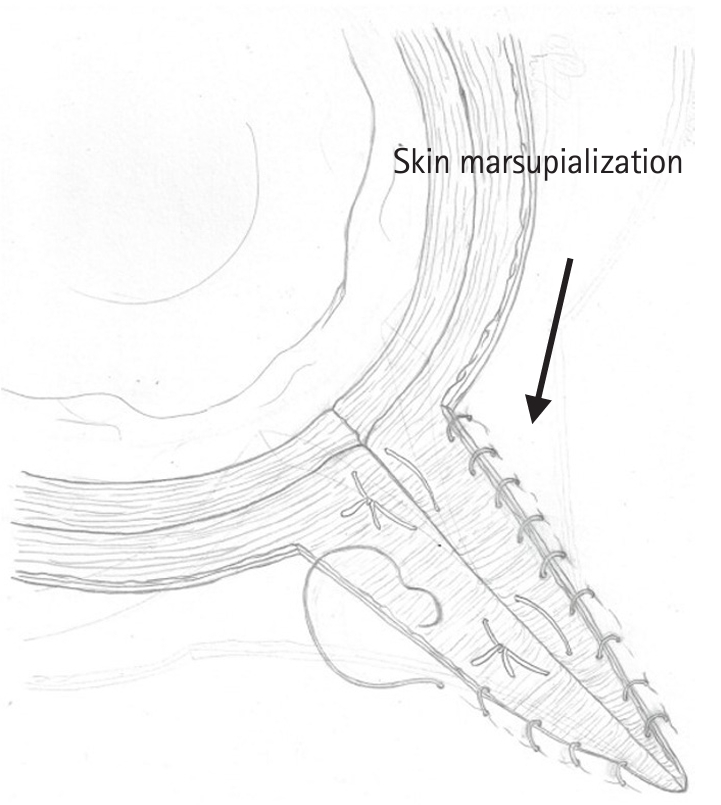
Fig. 1.
Fig. 2.
| Characteristic | Group A (n=45) | Group B (n=107) | P-value |
|---|---|---|---|
| Age (yr) | 46 (26–76) | 45 (22–72) | 0.16 |
| Sex | 0.20 | ||
| Male | 36 (80.0) | 95 (88.8) | |
| Female | 9 (20.0) | 12 (11.2) | |
| Type of fistula | <0.01 | ||
| Single | 33 (73.3) | 100 (93.5) | |
| Multiple | 12 (26.7) | 7 (6.5) | |
| Fistula classification | |||
| Intersphincteric | 2 (4.4) | 21 (19.6) | 0.02 |
| Low to mid transsphincteric | 20 (44.4) | 71 (66.4) | 0.02 |
| Mid to high transsphincteric | 17 (37.8) | 11 (10.3) | <0.01 |
| Suprasphincteric | 5 (11.1) | 4 (3.7) | 0.13 |
| Extrasphincteric | 1 (2.2) | 0 (0) | 0.29 |
| Prior incision and drainage | 28 (62.2) | 55 (51.4) | 0.28 |
| Prior fistula surgery | 21 (46.7) | 62 (57.9) | 0.22 |
| Baseline incontinence | 4 (8.9) | 7 (6.5) | 0.73 |
| Gas | 2 (50.0) | 4 (57.1) | |
| Liquid and/or solid stool | 2 (50.0) | 3 (42.9) |
| Outcome | Group A (n=45) | Group B (n=107) | P-value |
|---|---|---|---|
| Healing rate | 42 (93.3) | 97 (90.6) | 0.76 |
| Postoperative sepsis | 3 (6.7) | 4 (3.7) | 0.42 |
| New postoperative incontinence | 8 (17.8) | 15 (14.0) | 0.62 |
| Gas | 4 (50.0) | 6 (40.0) | |
| Liquid and/or solid stool | 4 (50.0) | 9 (60.0) | |
| Follow-up (wk) | 8 (1–77) | 8 (1–170) | 0.89 |
| Study | Study type | No. of patients | Mean follow-up (mo) | Healing rate (%) | Sphincter repair dehiscence (%) | Septic complication (%) | Postoperative continence disturbance (%) |
|---|---|---|---|---|---|---|---|
| Parkash et al. [24] (1985)] | Retrospective | 120 | 6–60 |
96.6 | 11.7 | - | 0 |
| Christiansen and Rønholt [25] (1995) | Prospective | 14 | 12–48 |
85.7 | - | - | 21.4 |
| Gemsenjäger [26] (1996) | Retrospective | 21 | 2–9 |
95.2 | 4.8 | - | 4.8 |
| Toccaceli et al. [27] (1997) | Retrospective | 36 | 12 | - | 8.2 | - | 0 |
| Roig et al. [28] (1999) | Retrospective | 31 | 24 |
90.3 | 3.2 | 3.2 | 24.0 |
| Perez et al. [29] (2005) | Prospective | 35 | 32 | 94.3 | 0 | 0 | 12.5 |
| Perez et al. [30] (2006) | RCT | 28 | 36 | 92.9 | 0 | 0 | 17.4 |
| Jivapaisarnpong [31] (2009) | Prospective | 33 | 14 | 87.9 | - | 6.1 | 0 |
| Roig et al. [32] (2010) | Retrospective | 75 | 13 | 89.4 | 1.3 | - | 21.3 |
| Kraemer and Picke [33] (2011) | Retrospective | 38 | - | 97.4 | 2.6 | - | 5.3 |
| Arroyo et al. [34] (2012) | Retrospective | 70 | 81 | 91.5 | 0 | 1.4 | 16.6 |
| Ratto et al. [35] (2013) | Retrospective | 72 | 29.4 | 95.8 | 1.4 | 0 | 11.6 |
| Hirschburger et al. [36] (2014) | Retrospective | 50 | 22 | 88.0 | - | - | 6.0 |
| Seyfried et al. [37] (2018) | Retrospective | 424 | 11 | 88.2 | 7.5 | - | 23.0 |
| Litta et al. [38] (2019) | Retrospective | 203 | 56 | 93.0 | 1.4 | 0 | 13.0 |
| Farag et al. [39] (2019) | Retrospective | 175 | 12 | 90.9 | - | - | 2.3 |
| De Hous et al. [40] (2021) | Retrospective | 24 | 6 |
95.8 | 25.0 | - | 20.8 |
| Aguilar-Martínez et al. [41] (2021) | Retrospective | 107 | 96 |
84.1 | - | - | 14.9 |
| Orban et al. [42] (2021) | Retrospective | 24 | 6 | 83.3 | 8.3 | 16.7 | 12.5 |
| Jain et al. [43] (2022) | Prospective | 35 | 6 | 88.6 | - | 20 | 5.7 |
| This study (2023) | Retrospective | 45 | 2b | 93.3 | 0 | 6.7 | 17.8 |
Values are presented as median (range) or number (%). Group A, patients who underwent fistulotomy with immediate primary sphincteroplasty. Group B, patients who underwent fistulotomy without sphincteroplasty.
Values are presented as number (%) or median (range). Group A, patients who underwent fistulotomy with immediate primary sphincteroplasty. Group B, patients who underwent fistulotomy without sphincteroplasty.
FIPS, fistulotomy with immediate primary sphincteroplasty; RCT, randomized controlled trial. Range. Median.
Table 1.
Table 2.
Table 3.
TOP
 KSCP
KSCP APFCP
APFCP